A large number of people are still contracting COVID-19. Fortunately, most of them are experiencing only mild symptoms, largely thanks to the high vaccination rate. However, in some individuals the disease takes a much more severe cause and our understanding about the underlying reasons is still insufficient. The human genome may hold a key to why COVID-19 is more serious for some people than others. A team of scientists from the MRC Epidemiology Unit and Berlin Institute of Health at Charité (BIH) together with other colleagues from Canada, Germany and the United Kingdom, have found genes and proteins that contribute to a higher risk of severe COVID-19. Their findings have now been published in the journal Nature Communications.
Doctors and scientists around the world are still in the dark as to why some people become severely ill when infected with SARS-CoV-2 (the virus that causes the COVID-19 disease), while others experience only mild symptoms. The team identified genes that – in addition known risk factors such as age and sex – predispose people to experience a more serious infection.
Dr Maik Pietzner of the the MRC Epidemiology Unit and BIH, and the study’s lead author, explained:
It has been observed relatively early on that susceptibility to infection depends on a person’s blood group, for example, which is inherited, so it was clear that the course of the disease is at least in part determined by genetics. But the causal genes and underlying mechanism remained unknown for many.”
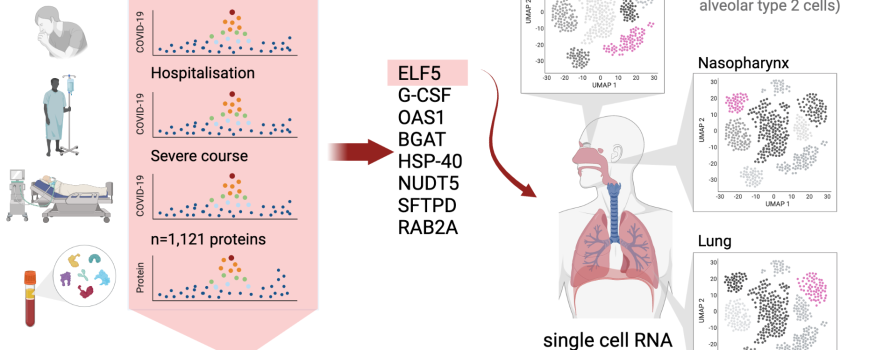
The team was given access to genetic data that researchers had collected from COVID-19 patients worldwide, which also documented disease progression. At the time their work began there were some 17 genomic regions observed to be associated with a higher risk of severe COVID-19 progression, but the causal genes and underlying mechanism remained unknown for many.
The Computational Medicine Group at BIH had previously developed a ‘proteogenomic’ approach to link protein-encoding regions of DNA to diseases via the protein product.
They applied this method to COVID-19 and came across eight particularly interesting proteins in this new study.
Dr Claudia Langenberg, also of the MRC Epidemiology Unit and head of the Computational Medicine Group at the BIH explained:
One of these was a protein responsible for an individual’s blood group, we were aware that this gene was associated with the risk of infection, so it was like a proof of concept. The protein ELF5, meanwhile, seemed like it could be much more relevant. We found that COVID-19 patients carrying a variant in the gene that encodes ELF5 were more much more likely to be hospitalized and ventilated, in some cases even died – so we took a closer look.”
The team turned to their colleagues from the Intelligent Imaging Group at BIH, led by Professor Christian Conrad, because of their expertise in single-cell analyses. Lorenz Chua, a doctoral student in the group was immediately enthusiastic to find out which cells displayed a particular abundance of the ELF5 protein:
We found that ELF5 is present in all surface cells of the skin and mucous membranes, but is produced in particularly large quantities in the lungs. Since this is where the virus causes most of its damage, this seemed very plausible.”
But Conrad puts a damper on any hopes that the scientists may have identified a new target molecule for drug development, explaining that:
ELF5 is what is known as a transcription factor, and controls how frequently or infrequently other genes are switched on and off throughout the body. Unfortunately, it is difficult to imagine interfering with this protein in any way, as that would undoubtedly cause many undesirable side effects.”
However, the scientists did find another interesting candidate among the eight suspects: the protein G-CSF, which serves as a growth factor for blood cells. They found that COVID-19 patients who genetically produce more G-CSF tend to experience a milder disease course. Synthetic G-CSF has been available as a drug for a long time, so its use as a treatment for COVID-19 patients could be conceivable.
Translation of such genetic discoveries into clinical application is not an easy or rapid process. The work – only possible through the support of many scientists and clinicans, and open access results from studies around the world – highlights how open science and an international team effort can step by step uncover how the smallest changes in our genetic make-up alter the course of disease, COVID-19 in this example. Pietzner concludes:
We started with global data from 100,000 participants and ended up looking at single molecules in individual cells. We believe that collaborations that allow us to rapidly move from the bigger picture and studying large populations to in depth molecular follow-up can help to better understand the clinical consequences of this virus and teach us important lessons for future pandemics.”
- Pietzner, M et al. ELF5 is a respiratory epithelial cell-specific risk gene for severe COVID-19. Nature Communications; 15 Aug 2022; DOI: 10.1038/s41467-022-31999-6

 MRC Epidemiology Unit
MRC Epidemiology Unit